[Source: Reuters]
India has more work to do in halting sales of toxic cough syrup, despite some progress, a World Health Organization official told Reuters, after at least 24 children died following consumption of a domestically-made medicine.
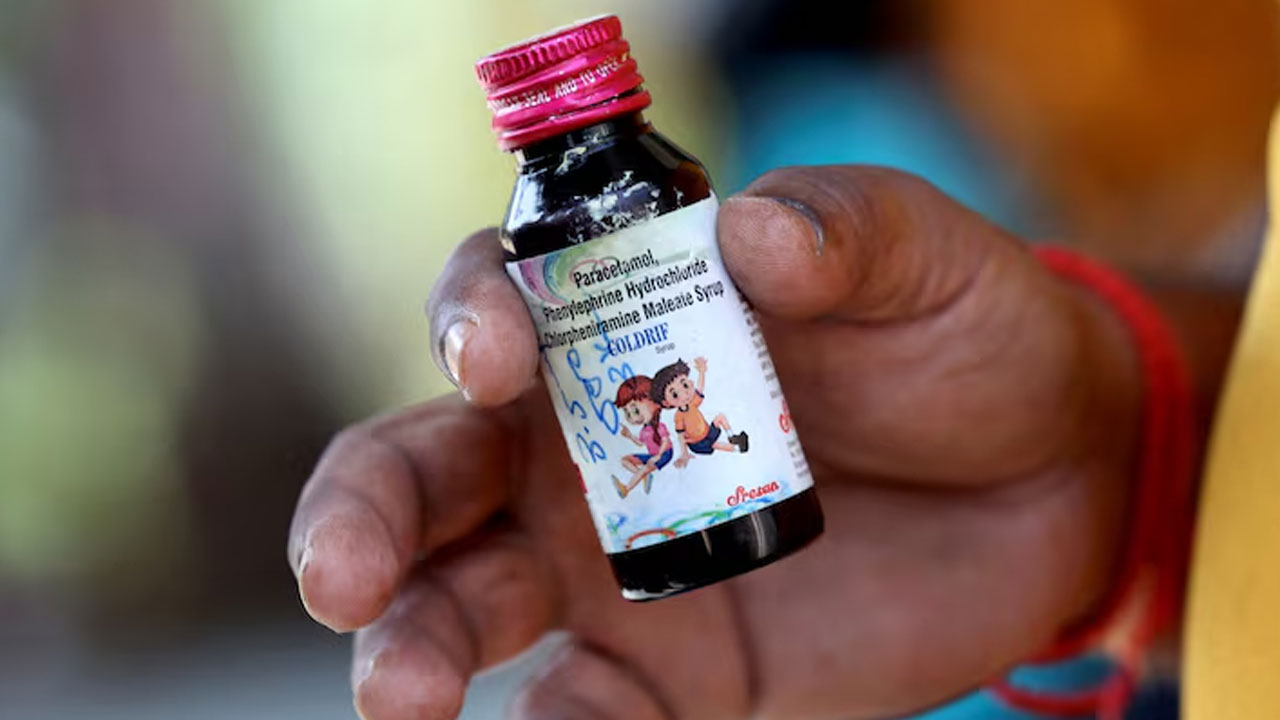
The children died after taking the Coldrif cough medicine made by Sresan Pharma, which tests showed contained the toxin diethylene glycol in quantities nearly 500 times the permissible limit.
They came just two years after global pledges to tighten the system following the deaths of at least 300 children around the world linked to similar toxins in syrup-based medicines made in India and Indonesia.
But enforcement issues persist, the WHO said.
“They have made some strides,” said the official, Rutendo Kuwana, referring to a new Indian rule requiring medicine to be tested for contaminants like diethylene and ethylene glycol before export.
However, no such rule exists for syrups sold locally – a “regulatory gap” the WHO has flagged.
“It’s a work in progress,” added Kuwana, the WHO team lead for incidents involving substandard and falsified medicines.
“There’s a lot that needs to be done. It’s a big market, with tens of thousands of manufacturers and many states to deal with.”
Last week Reuters reported that India plans to scrap its export rule once companies upgrade their facilities to international standards by a year-end deadline.
India’s health ministry and the Central Drugs Standard Control Organisation (CDSCO), the federal pharmaceuticals regulator, did not return requests for comment on the plans.
Representatives of Sresan Pharma did not respond to repeated telephone calls.
A WHO spokesperson said by email on Monday the agency welcomed all steps to improve medicine quality.
However, in response to a question about dropping the export tests, the spokesperson said medicines, including raw materials, should be tested throughout the production process, not just at the end.
Stream the best of Fiji on VITI+. Anytime. Anywhere.


 Reuters
Reuters