[Source: AP]
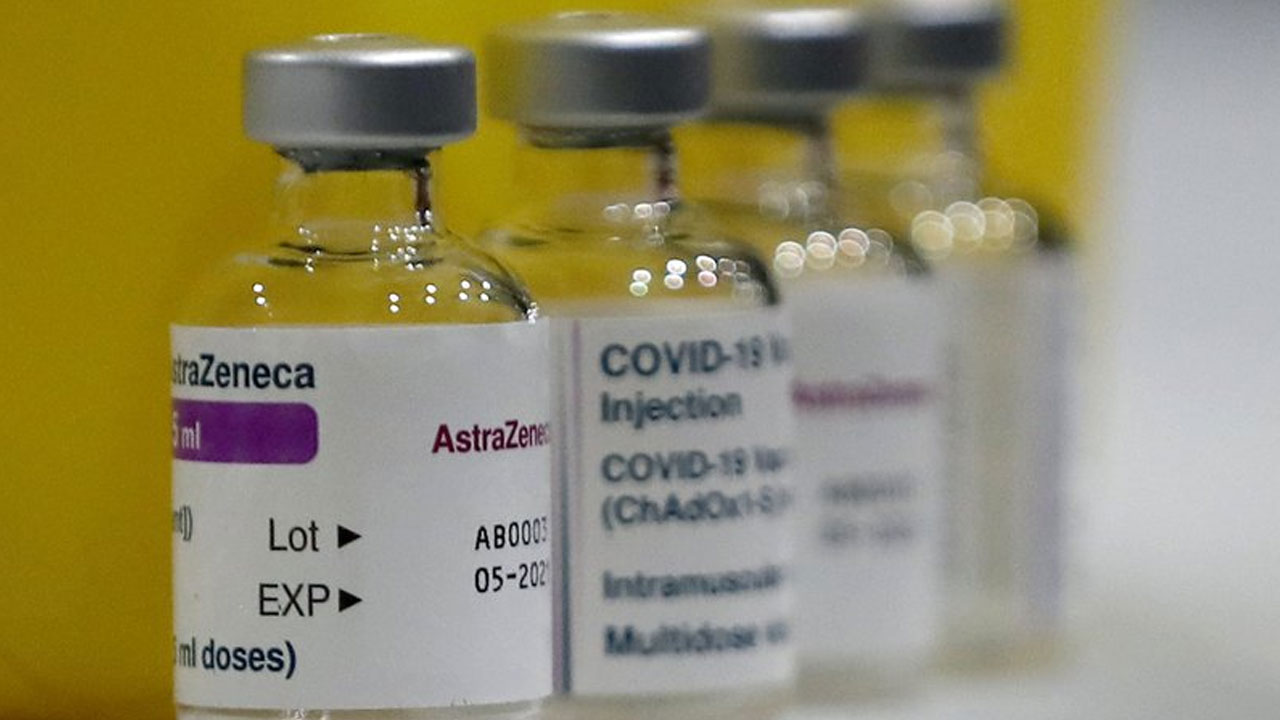
AstraZeneca’s COVID-19 vaccine shows a hint that it may reduce transmission of the virus.
It offers strong protection for three months on just a single dose, researchers said Wednesday in an encouraging turn in the campaign to suppress the outbreak.
The preliminary findings from Oxford University, a co-developer of the vaccine, could vindicate the British government’s controversial strategy of delaying the second shot for up to 12 weeks so that more people can be quickly given a first dose. Up to now, the recommended time between doses has been four weeks.
The research could also bring scientists closer to an answer to one of the big questions about the vaccination drive: Will the vaccines actually curb the spread of the coronavirus?
It’s not clear what implications, if any, the findings might have for the two other major vaccines being used in the West, Pfizer’s and Moderna’s.
In the United States, Dr. Anthony Fauci, the nation’s top infectious disease expert, dismissed the idea of deliberately delaying second shots, saying the U.S. will “go by the science” and data from the clinical trials. The two doses of the Pifzer and Moderna vaccines are supposed to be given three and four weeks apart.
Still, the research appears to be good news in the desperate effort to arrest the spread of the virus and also suggests a way to ease vaccine shortages and get shots into more arms more quickly.
The makers of all three vaccines have said that their shots proved to be anywhere from 70% to 95% effective in clinical trials in protecting people from illness caused by the virus. But it was unclear whether the vaccines could also suppress transmission of the virus — that is, whether someone inoculated could still acquire the virus without getting sick and spread it to others.
As a result, experts have been saying that even people who have been vaccinated should continue to wear masks and keep their distance from others.
Volunteers in the British study underwent regular nasal swabs to check for the coronavirus, a proxy to try to answer the transmission question. The level of virus-positive swabs — combining volunteers who had asymptomatic infection with those who had symptoms — was 67% lower in the vaccinated group, the researchers reported.
While not a direct measure, “that’s got to have a really beneficial effect on transmission,” Oxford lead researcher Sarah Gilbert told a meeting of the New York Academy of Sciences Wednesday.
The researchers also looked at how likely people who have been vaccinated are to get a symptom-free infection. In one subset of volunteers, there were 16 asymptomatic infections among the vaccinated and 31 in an unvaccinated comparison group.
Pfizer and Moderna also are studying the effect of their vaccines on asymptomatic infections.
Only the Pfizer and Moderna vaccines are being used in the United States. Britain is using both AstraZeneca’s and Pfizer’s. AstraZeneca’s has also been authorized by the 27-nation European Union. Pfizer has not endorsed the British government’s decision to lengthen the time between doses.
Mene Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said that no patients experienced severe COVID-19 or required hospitalization three weeks after receiving a first dose, and that effectiveness appeared to increase up to 12 weeks after the initial shot.
“Our data suggest you want to be as close to the 12 weeks as you can” for the second dose, Pangalos said.
British Health Secretary Matt Hancock said the study “backs the strategy that we’ve taken” to make sure more people have gotten at least one shot. Britain’s decision has been criticized as risky by other European countries.
Stephen Evans of the London School of Hygiene and Tropical Medicine said the study’s suggestion that a single dose protected people for 12 weeks was “useful but not definitive.”
He said that the authors themselves acknowledged their research was not designed to investigate the vaccine’s dosing schedule and that their conclusions were based on statistical modeling, not actual patients tracked over time.
“It certainly isn’t very strong evidence, but there is also no indication this is the wrong thing to do,” Evans said of Britain’s strategy.